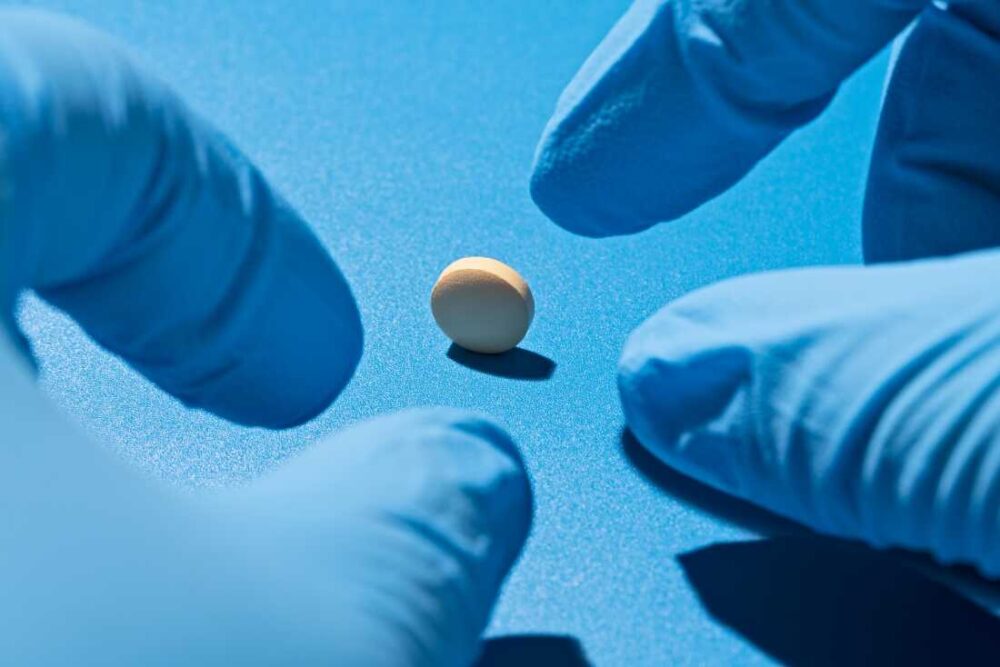
The world of psychedelic medicine faces a significant turning point as developers grapple with the recent rejection of an ecstasy-based treatment by the FDA. This decision has sent ripples through the industry, signaling potential challenges ahead for companies involved in the development of psychedelic therapies.
Key Highlights from the FDA Rejection:
- The FDA expressed concerns regarding the safety and efficacy of the ecstasy-derived drug.
- Regulatory scrutiny is anticipated to intensify for psychedelic drug developers.
- Despite this setback, many companies remain dedicated to advancing psychedelic research.
This rejection marks a crucial moment for psychedelic drug research, which has garnered growing attention due to its potential in treating mental health disorders such as PTSD and depression. As companies prioritize compliance and work to address regulatory feedback, industry stakeholders await further developments.
With the landscape shifting, it is paramount for developers to adapt and innovate. Regulatory frameworks are evolving, and adept navigation of these challenges may open new avenues for success in the future.
Future Outlook:
- Increased collaboration between developers and regulatory agencies may facilitate progress.
- Continued investment in research could lead to breakthroughs in psychedelic medicine.
- The public and scientific communities remain optimistic about the potential of psychedelics.
As psychedelic drug developers assess their strategies in light of the FDA’s decision, there remains a firm belief in the therapeutic potential of these substances. The journey may face hurdles, but the pursuit of innovation continues, as many stakeholders rally towards a future where psychedelics unlock new possibilities for healing.








Leave a Reply