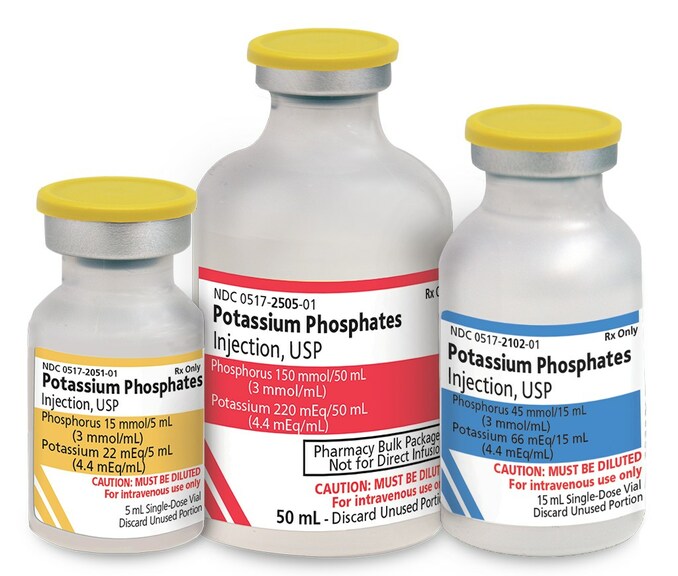
Amneal Pharmaceuticals has announced that it has received FDA approval for its potassium phosphate intravenous (IV) bags, marking a significant achievement in its pharmacological offerings. This approval comes at a crucial time when the healthcare industry is seeking effective solutions for patients needing nutrient replenishment through IV therapy. Key Highlights:
With the increasing demand for potassium supplementation in clinical settings, Amneal’s potassium phosphate IV bags will not only enhance clinical outcomes but also align with the growing focus on high-quality patient care. Moreover, this approval may position Amneal favorably in the competitive landscape, as healthcare providers continuously search for dependable and effective solutions for their patients. The company is expected to leverage this opportunity to broaden its market reach and support healthcare professionals in providing comprehensive care. In summary, the FDA’s approval of Amneal’s potassium phosphate IV bags signifies a positive step forward in addressing the critical need for electrolyte management in medical settings, ultimately benefiting both the healthcare industry and patients alike.








Leave a Reply