UCB’s Commitment to Psoriasis Care
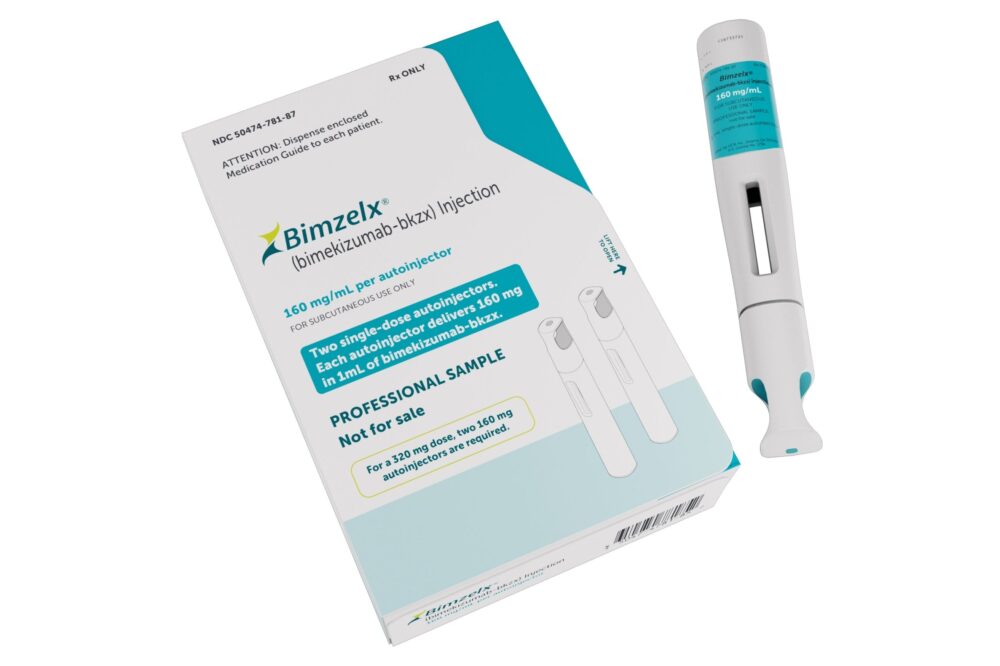
UCB, a global biopharmaceutical company, has recently received expanded FDA approval for its innovative psoriasis treatment, Bimzelx. This groundbreaking development is a significant step forward for patients living with this chronic skin condition, as Bimzelx now offers even more comprehensive options for management and care.
What Makes Bimzelx Stand Out?
– **New Indications**: The expanded approval allows healthcare providers to prescribe Bimzelx for additional patient groups, enhancing its accessibility.
– **Proven Efficacy**: Clinical trials have demonstrated Bimzelx’s effectiveness in reducing psoriasis symptoms, leading to improved quality of life for users.
– **Safety Profile**: UCB maintains a strong emphasis on patient safety, with Bimzelx showing a manageable safety profile during trials.
Impacts on Patient Care
The FDA’s endorsement of Bimzelx is a monumental achievement for UCB and a boon for individuals suffering from moderate to severe plaque psoriasis. With its dual mechanism of action, Bimzelx not only addresses the skin manifestations of psoriasis but also aims to manage underlying inflammation, marking a holistic approach to treatment.
Future Prospects for UCB and Psoriasis Patients
UCB’s ongoing commitment to research and patient care signifies a hopeful future for those affected by psoriasis. As doctors gain more tools like Bimzelx to combat this complex disease, patients can look forward to improved treatment experiences and outcomes.
Stay tuned for more updates as UCB continues to revolutionize psoriasis treatment with innovative solutions like Bimzelx. Those interested in learning more should consult with their healthcare providers about the suitability of this medication for their situations. In the realm of dermatology, UCB’s advancements herald a new era of possibilities for effective psoriasis management.








Leave a Reply